Semi-synthesis in the Exploration of Opioid-targeting Natural Products
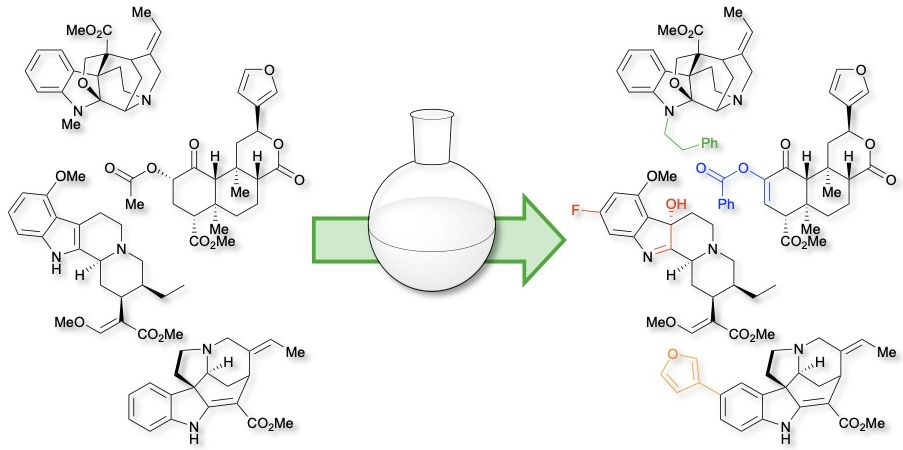
Riley, A. P. Semi-synthesis in the Exploration of Opioid-targeting Natural Products, Natural Product Reports, In Press. Link to Article.
Comprehensive Pharmacokinetics and ADME Evaluation of Akuamma Alkaloids
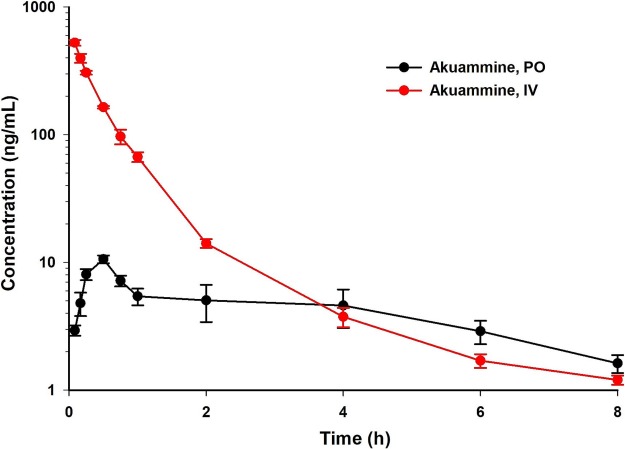
Gour, A.; Creed, S. M.; Riley, A. P. Sharma, A. Comprehensive Pharmacokinetics and ADME Evaluation of Akuamma Alkaloids, Journal of Chromatography B, 2025, 1263, 124713. Link to Article.
Pharmacological Characterization of the Novel Selective Kappa Opioid Receptor Agonists 10-Iodo-Akuammicine and 10-Bromo-Akuammicine in Mice
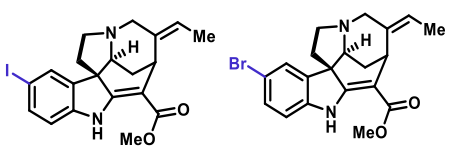
Bland, K; Chen, C.; Huang, P.; Ho, C.; Howe, T.; Ocampo, K.; Zhao, P.; Creed, S.; Noel-Torres, J.; Riley, A. P. Liu-Chen, L-Y. Pharmacological Characterization of the Novel Selective Kappa Opioid Receptor Agonists 10-Iodo-Akuammicine and 10-Bromo-Akuammicine in Mice, Neuropharmacology, 2025, 268, 110316. Link to Article.
Derivatives of Aristoquinoline Accessed through a Ritter-like Reaction
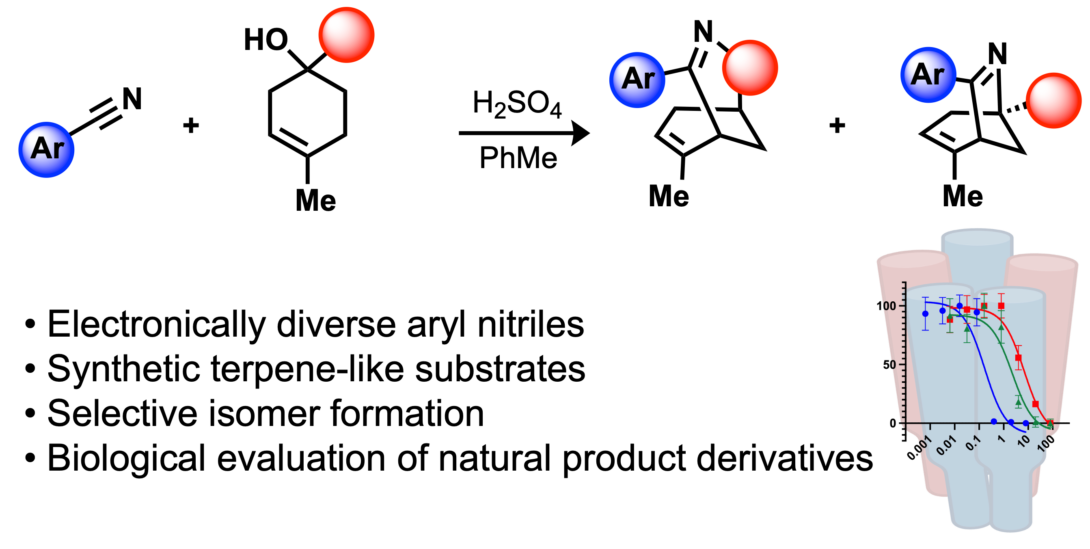
Straub, C. J.; Rusali, L. E.; Riley, A. P. Derivatives of Aristoquinoline Accessed through a Ritter-like Reaction, ACS Medicinal Chemistry Letters, 2025, 16, 406-409. Link to Article.
Featured on the Front Cover of ACS Medicinal Chemistry Letters.
Discovery of Potent Kappa Opioid Receptor Agonists Derived from Akuammicine
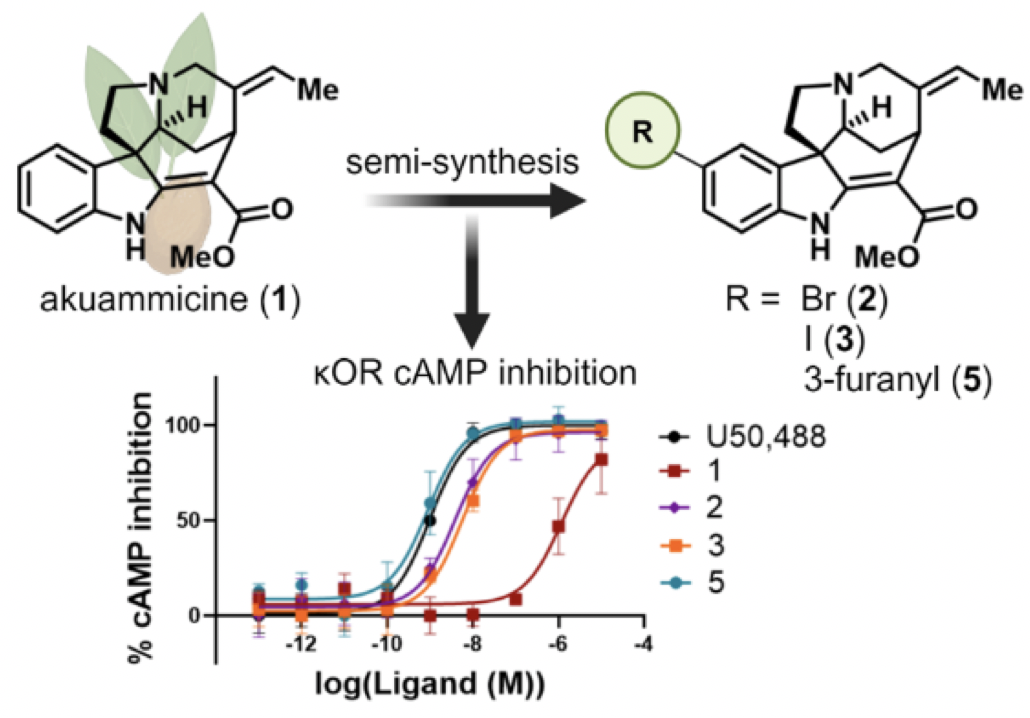
Hennessy, M. R.; Creed, S. M.; Gutridge, A. M.; Rusali, L. E.; Lou, D.; Sepehri, B.; Rhoda, E. S.; Villegas, J. A.; van Rijn, R. M.; Riley, A. P. Discovery of Potent Kappa Opioid Receptor Agonists Derived from Akuammicine, Journal of Medicinal Chemistry, 2024, 67, 20842–20857. Link to Article.
Discovery of Antinociceptive α9α10 Nicotinic Acetylcholine Receptor Antagonists by Stable Receptor Expression
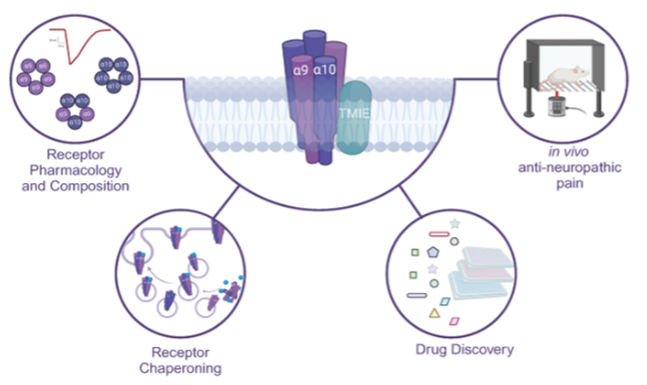
Kremiller, K. M.; Kulkarni, G. C; Harris, L. M.; Gunasekara, H.; Kashyap, Y.; Ilktach, G. Nguyen, A.; Ondrus, A. E.; Hu, Y. S.; Wang, Z. J.; Riley, A. P.*; Peters, C. J.* Discovery of Antinociceptive α9α10 Nicotinic Acetylcholine Receptor Antagonists by Stable Receptor Expression, ACS Chemical Biology, 2024, 19, 2291–2303. Link to Article.
Synthesis of α3β4 Nicotinic Acetylcholine Receptor Modulators Derived from Aristoquinoline that Reduce Reinstatement of Cocaine-Seeking Behavior
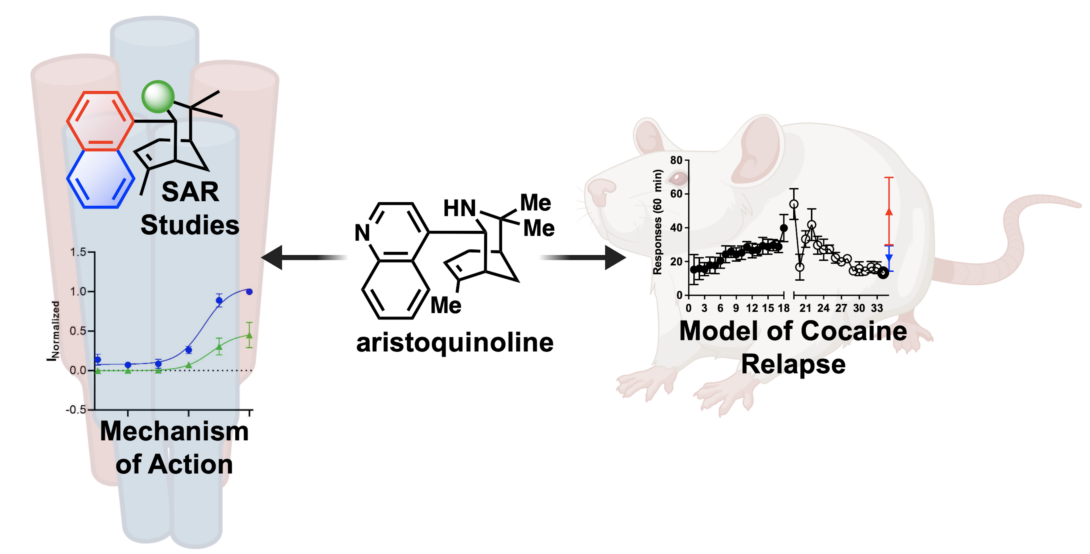
Rusali, L. E.*; Lopez-Hernandez, A. M.*; Kremiller, K. M.; Kulkarni, G. C.; Gour, A.; Straub, C. J.; Argade, M. D.; Peters, C. J.; Sharm, A.; Toll, L.; Cippitelli, A.; Riley, A. P. Synthesis of α3β4 Nicotinic Acetylcholine Receptor Modulators Derived from Aristoquinoline that Reduce Reinstatement of Cocaine-Seeking Behavior. J. Med. Chem. 2024, 67, 529–542. Link to Article.
*Authors contributed equally to this work.
Featured on the Cover of the Journal of Medicinal Chemistry.
Modified Akuamma Alkaloids with Increased Potency at the Mu-opioid Receptor
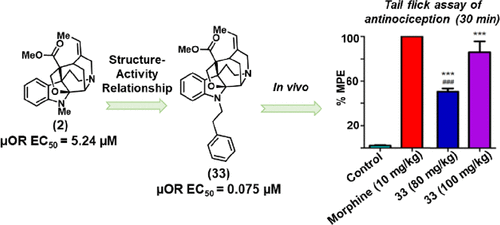
Hennessy, M. R.*; Gutridge, A. M*; French, A. R.; Rhoda, E. S.; Meqbil, Y. J.; Gill, M.; Kashyap, Y.; Appourchaux, K.; Paul, B.; Wang, Z. J.; van Rijn, R. M.; Riley, A. P. Modified Akuamma Alkaloids with Increased Potency at the Mu-opioid Receptor. J. Med. Chem. 2023, 66, 3312–3326. Link to Article.
*Authors contributed equally to this work.
What We Have Gained from Ibogaine: α3β4 Nicotinic Acetylcholine Receptor Inhibitors as Treatments for Substance Use Disorders
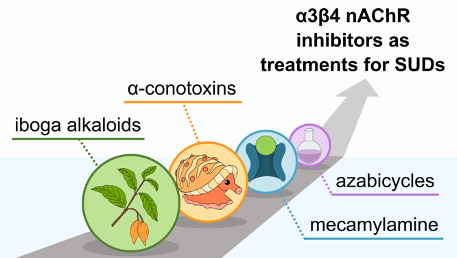
Straub, C. J.; Rusali, L. E.; Kremiller, K. M.; Riley, A. P. What We Have Gained from Ibogaine: α3β4 Nicotinic Acetylcholine Receptor Inhibitors as Treatments for Substance Use Disorders. J. Med. Chem. 2023, 66, 107-121. Link to Article.
Featured on the Front Cover of the Journal of Medicinal Chemistry.
Syntheses of Aristotelia Alkaloids: Reflections in the Chiral Pool
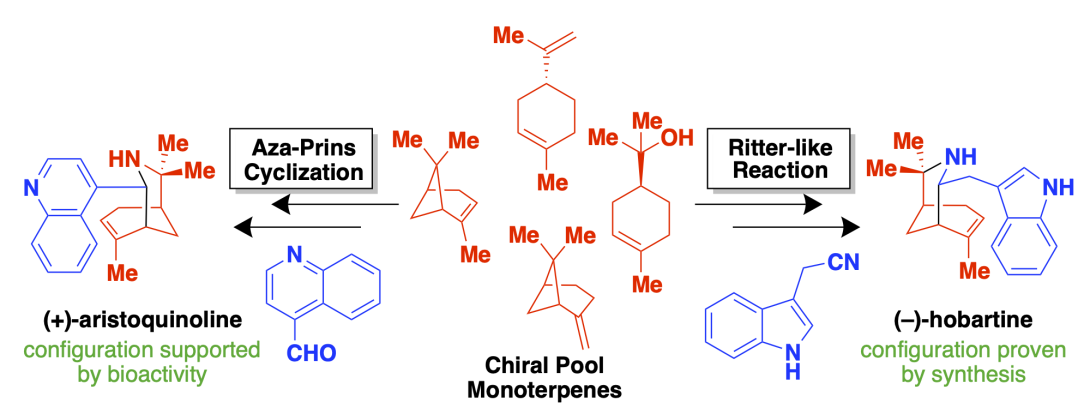
Argade, M. D.; Riley, A. P. Syntheses of Aristotelia Alkaloids: Reflections in the Chiral Pool. Synlett 2022, 33, 1209-1214. Link to Article.
Synthesis of Aristoquinoline Enantiomers and Their Evaluation at the α3β4 Nicotinic Acetylcholine Receptor
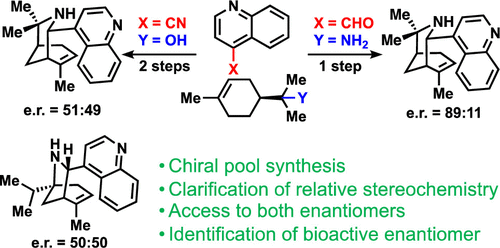
Argade, M. D.; Straub, C. J.; Rusali, L. E.; Santarsiero, B. D.; Riley, A. P. Synthesis of Aristoquinoline Enantiomers and Their Evaluation at the α3β4 Nicotinic Acetylcholine Receptor. Org. Lett. 2021, 23, 7693-7697. Link to Article
Featured on the Front Cover of Organic Letters.
Featured in Synfacts.
Isolation and Pharmacological Characterization of Six Opioidergic Picralima nitida Alkaloids
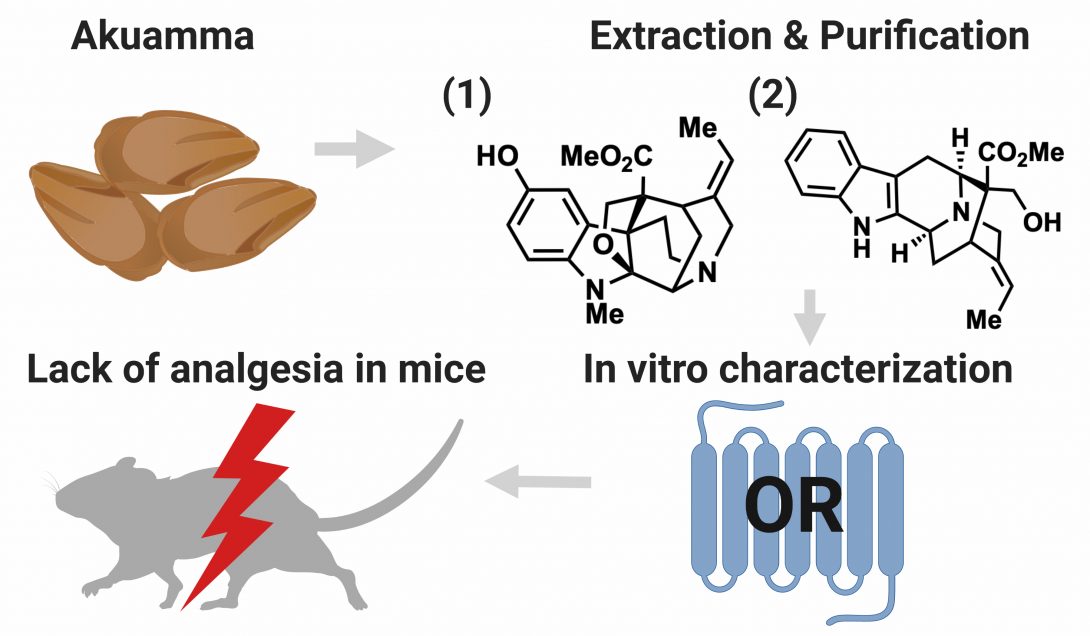
Creed, S.;* Gutridge, A.;* Argade, M. D.; Hennessy, M.; Friesen, J. B.; Pauli, G. F.; van Rijn, R.; Riley, A. P. Isolation and Pharmacological Characterization of Six Opioidergic Picralima nitida Alkaloids. J. Nat. Prod. 2021, 84, 71-80. Link to Article
*Authors contributed equally to this work.
Prior to UIC
- Shelton, C. L.; Meneely, K. M.; Ronnebaum, T. A.; Chilton, A. S.; Riley, A. P.; Prisinzano, T. E.; Lamb, A. L. Rational Inhibitor Design for Pseudomonas aeruginosa Salicylate Adenylation Enzyme PChD, J. Biol. Inorg. Chem. 2022, 27, 541-551. Link To Article
- Crowley, R. S.; Riley, A. P.; Alder, A. F.; Anderson, R. J.; Luo, D.; Kaska, S.; Maynez P.; Kivell, B. M.; Prisinzano, T. E. Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Design, Synthesis, and Evaluation of Analogues with Improved Potency and G-protein Activation Bias at the μ-Opioid Receptor, ACS Chem. Neurosci. 2020, 11, 1781-1790. Link to Article
- Richter, M. F.; Drown, B. S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R. L.; Hergenrother, P. J. Predictive Compound Accumulation Rules Yield a Broad-spectrum Antibiotic, Nature, 2017, 545, 299-304. Link to Article
- Crowley R. S.*; Riley A. P.*; Sherwood A. M.; Groer C.E.; Shivaperumal N.; Biscaia M.; Paton K.; Schneider S.; Provasi D.; Kivell B. M.; Filizola M.; Prisinzano T. E. Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Identification of a Potent and Centrally Acting μ-Opioid Analgesic with Reduced Abuse Liability, J. Med. Chem. 2016, 59, 11027-11038. *Authors contributed equally to this work Link to Article
- Meneely, K. M.; Ronnebaum, T. A.; Riley, A. P.; Prisinzano, T. E.; Lamb, A. L. Holo Structure and Steady State Kinetics of the Thiazolinyl Imine Reductases for Siderophore Biosynthesis, Biochemistry, 2016, 55, 5423-5433. Link to Article
- Paranjape S. R.; Riley, A. P.; Somoza, A. D.; Oakley, C. E.; Wang, C. C.; Prisinzano, T. E. Oakley, B. R.; Gamblin, T. C. Azaphilones Inhibit Tau Aggregation and Dissolve Tau Aggregates In Vitro, ACS Chem. Neurosci. 2015,6,751-760. Link to Article
- Riley, A. P.; Groer, C. E.; Young, D.; Ewald A. W. Kivell, B. M. Prisinzano, T. E. Synthesis and κ-Opioid Receptor Activity of Furan-substituted Salvinorin A Analogues. J. Med. Chem. 2014, 57, 10464-10475. Link to Article
- Meneely, K. M.; Luo, Q.;Riley, A. P.; Taylor, B.; Roy, A.; Stein, R. L.; Prisinzano, T. E.; Lamb, A. L. Expanding the Results of a High Throughput Screen Against an Isochorismate-pyruvate Lyase to Enzymes of a Similar Scaffold or Mechanism. Biorg. Med. Chem. 2014, 22, 5961-5969. Link to Article
- Riley, A. P.; Day, V. W.; Navarro, H. A.; Prisinzano, T. E. Palladium-catalyzed Transformations of Salvinorin A, A Diterpene from Salvia divinorum. Org. Lett. 2013, 15, 5936-5939. Link to Article