Riley Lab Research Heading link
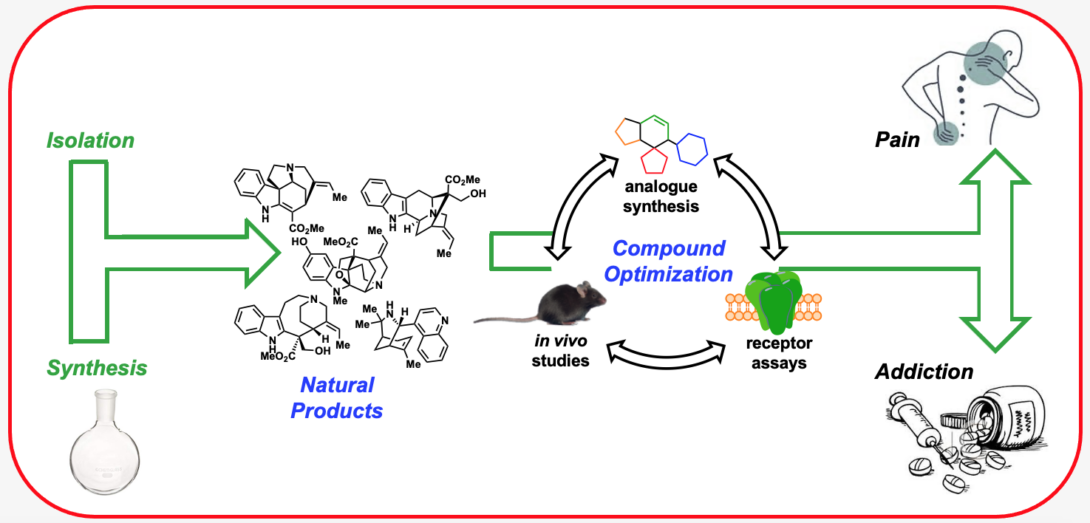
Research in the Riley Lab centers on the discovery and development of novel compounds that target ligand-gated ion channels (LGICs) and G-protein-coupled receptors (GPCRs) involved in pain and addiction. In doing so, we aim to provide highly potent and selective chemical tools to probe the pharmacology of these important receptor targets and provide promising drug leads. To accomplish our goals, we are often draw inspiration from the chemical complexity provided by nature in the form of alkaloid natural products. Due in large part to their unmatched structural complexity and unique ability to perturb biological pathways, natural products have historically served as excellent starting points in the drug discovery process. However, in many instances natural products lack the appropriate selectivity or drug-like properties to be useful as chemical probes or medicine. To address these concerns, we employ the techniques of medicinal chemistry including organic chemistry, receptor assays, computational chemistry, and structural biology, to study and optimize the activity of several classes of natural products. We are currently applying this multidisciplinary approach to study the opioid and acetylcholine receptors.
Akuamma Alkaloid Heading link

The akuamma alkaloids are a class of natural products found in the seeds of the akuamma tree (Picralima nitida), which have been used in traditional African medicine for chronic pain. Driven in by these anecdotal reports of the analgesic activity, the objective of this project is to elucidate the biological target(s) of the akuamma alkaloids and determine how they can be translated into safer, more effective pain medicine. After developing a robust process to isolate large quantities of the akuamma alkaloids, we identified two alkaloids, akuammine and akuammicine, that activated the mu and kappa opioid receptors (µOR and κOR), respectively. Because these compounds are structurally distinct from traditional opioid ligands, we are determining how structural modifications to the chemical structure of akuammine and akuammicine impact their activity at the opioid receptors. Through this process we have identified several highly potent and selective κOR and µOR ligands, which through collaboration, we are advancing into various models of pain and addiction.
a3b4 nAChRs Heading link
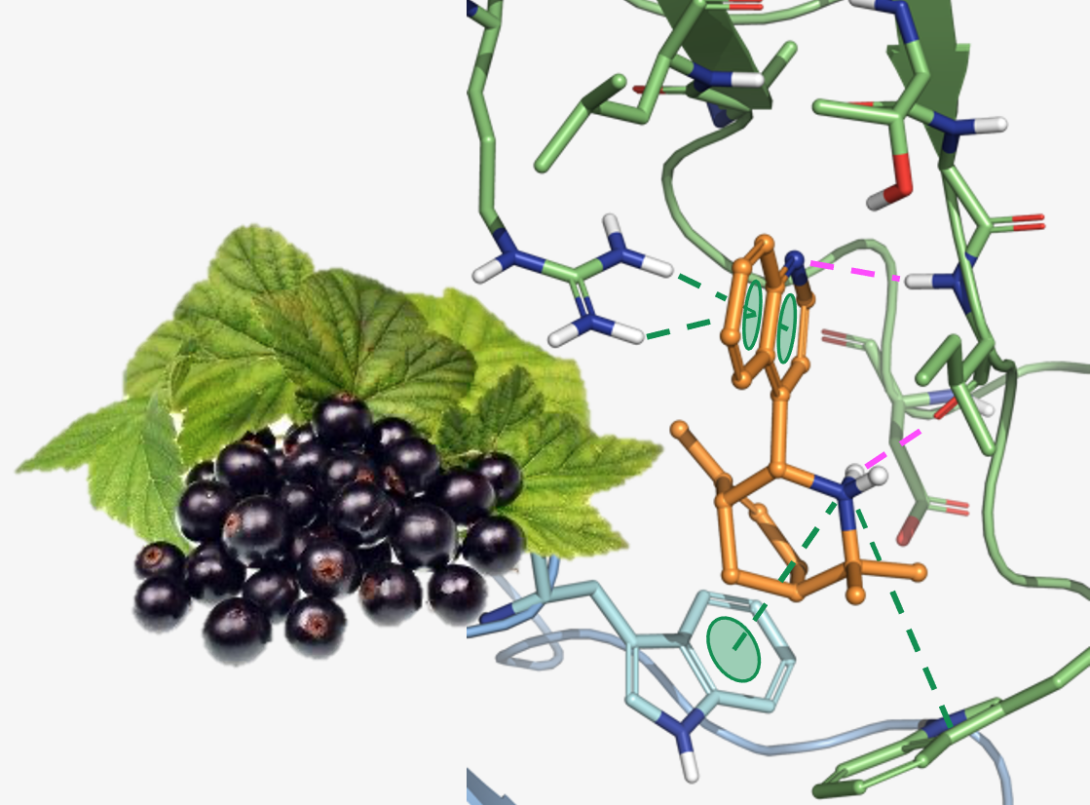
The Aristotelia alkaloids are a family of monoterpene indole alkaloids present in the leaves of numerous plants from the Aristotelia family. Work by our lab and others has identified several of these Aristotelia alkaloids as inhibitors of the α3β4 nicotinic acetylcholine receptor (nAChR). The nicotinic acetylcholine receptors (nAChRs) are a family of ligand-gated ion channels found throughout the peripheral and central nervous systems, and the α3β4 subtype has been implicated as a target to treat a variety of substance-use disorders (i.e., drug addictions). By developing a highly modular synthesis of the Aristotelia alkaloid scaffold and implementing a state-of-the-art receptor assay, we have designed several highly selective α3β4 inhibitors. Through mechanism-of-action studies, we have recently determined these compounds bind to an allosteric site, which we believe is responsible for the observed subtype-selectivity. In collaboration with Dr. Lawrence Toll at Florida Atlantic University, we have also shown that these compounds significantly block the reinstatement of cocaine-seeking behavior in an animal model of drug relapse. We are now exploring the translational potential for these α3β4 negative allosteric modulators as treatments for cocaine-use disorder and other substance use disorders.